When Ide Mills first learned about the Cancer Support Community Registry, she jumped at the chance to participate. Mills, who has stage 4 lung cancer, went online at cancerexperienceregistry.org to answer demographic questions and in-depth queries about her experience with her illness, such as what worried her the most and what her everyday challenges were in living with her disease.
The registry collects information about the social and emotional experiences of cancer patients and their caregivers. Then, Cancer Support Community, a nonprofit patient advocacy group, uses the data to improve its own programs and to help enhance research and care programs offered by the health care community.
 For IDE MILLS, joining a patient registry was not just about helping researchers gather data, but about feeling like part of a community. – PHOTO BY DIANA LEE
For IDE MILLS, joining a patient registry was not just about helping researchers gather data, but about feeling like part of a community. – PHOTO BY DIANA LEE
For Mills, of Maplewood, New Jersey, filling out the online form represented a chance to help someone else have a better cancer journey, but since then, the registry has come to mean even more to her. Mills not only participates actively in the registry, but is an advisor on one of its panels. She and other members of this community contribute to the study of trends, share their thoughts about research findings and help design programs that meet the needs of patients and caregivers.
“It’s important for patients to know they are not alone, and cathartic to share one’s story,” Mills says. “Most important, being a part of a larger community bonded by similar life experiences is invaluable.”
Registries are designed for the collection of data, but, unlike clinical trials, they are not designed to test drugs under controlled conditions; rather, registries look at established standards of care and choices made by patients and their doctors during the normal course of treatment. This means that registries answer different questions than clinical trials do. While a trial estimates the safety and/or effectiveness of a treatment, a registry determines whether a new treatment is being appropriately used in a population group and whether the benefits found in clinical trials are consistent with what is being observed in a more diverse population. In some cases, information is collected about a specific disease or clinical situation, rather than a specific therapy. Registries can also look for trends in cancer incidence that might be addressable through changes in health care practices. …
Read Full Article (Excerpt of Article from CureToday.com)

Rebecca Cassady, RHIA, CTR Director
Desert Sierra Cancer Surveillance Program Region 5 of Cancer Registry of Greater California
(NAACCR Committee Member)
‘The Cancer Registry Rationale’ illustrates the impact of cancer registries on public health and patient care. Utilizing the data that is obtained can provide the impetus to evaluate treatment patterns, influence cancer prevention measures and provide resources for scientific analyses. Specialized cancer registries have been developed to provide additional focus and generate support resources to improve patient outcomes. The continuous work of the cancer surveillance community contributes to the overarching goal of reducing the burden of cancer on society and improving the quality of life for cancer patients.
The opinions expressed in this article are those of the authors and may not represent the official positions of NAACCR.

 The
The 



 Highlights from the report were presented at the NAACCR 2016 Annual Conference and include:
Highlights from the report were presented at the NAACCR 2016 Annual Conference and include:

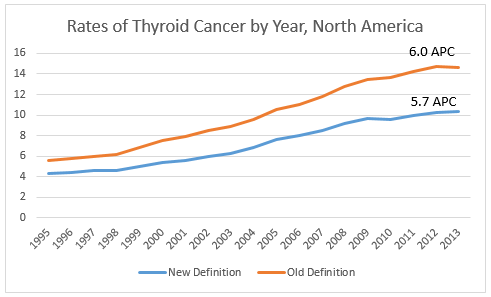 In North America, reportablility follows the guidelines established by the WHO. We will be monitoring WHO “blue books” to see if this reclassification is officially adopted. In order to effectively track the burden of thyroid cancer, the cancer surveillance community will need to ensure our current classification system can accommodate this change, develop guidelines for assessing thyroid cancer trends over time, and educate the public about what is driving the significant drop in thyroid cancer incidence we will see in the new few years.
In North America, reportablility follows the guidelines established by the WHO. We will be monitoring WHO “blue books” to see if this reclassification is officially adopted. In order to effectively track the burden of thyroid cancer, the cancer surveillance community will need to ensure our current classification system can accommodate this change, develop guidelines for assessing thyroid cancer trends over time, and educate the public about what is driving the significant drop in thyroid cancer incidence we will see in the new few years. Thomas C. Tucker, PhD, MPH, Associate Director for Cancer Control and Director of the Kentucky Cancer Registry University of Kentucky Markey Cancer Center (NAACCR Steering Committee Chair, Past-President)
Thomas C. Tucker, PhD, MPH, Associate Director for Cancer Control and Director of the Kentucky Cancer Registry University of Kentucky Markey Cancer Center (NAACCR Steering Committee Chair, Past-President) A. Blythe Ryerson, MPH, PHD, Interim Chief, Cancer Surveillance Branch, Centers for Disease Control and Prevention (NAACCR Committee Member)
A. Blythe Ryerson, MPH, PHD, Interim Chief, Cancer Surveillance Branch, Centers for Disease Control and Prevention (NAACCR Committee Member)