
Recinda L Sherman, MPH, PhD, CTR Program Manager, Data Use & Research North American Association of Central Cancer Registries
(217) 698-0800 x 6; rsherman@naaccr.org
While there are some well established carcinogens, for example tobacco, the largest risk for most cancers is age. And while we understand that individual-level lifestyle factors have a significant impact on cancer incidence, like diet; it is also clear that contextual factors such as poverty and regional policies influence cancer risk and outcomes. We can rightfully highlight public health successes in tobacco cessation and subsequent decline in tobacco-related cancers, as well as other tobacco-affiliated disease. But because cancer has a long latency period and is multicausal, primary prevention efforts are hampered by our limited understanding of what causes most cancers.
This places additional reliance on secondary prevention efforts, primarily screening and effectively treating early stage cancers, to address the burden of cancer. For some cancers, there are effective, population-based screenings, such as for cervical and colorectal cancer. And new advances in low-dose CT scanning are important secondary prevention tools for lung cancer by screening high-risk individuals, based on age and smoking history. Other important secondary prevention efforts include improvements in diagnostic screening tools, intended to screen symptomatic individuals versus the general public, which are also important in influencing stage-shift.
But the focus on detecting early stage cancers is not universally positive and is leading to overdiagonosis, particulary for prostate, breast, melanoma, and thyroid cases. Overdiagnosis is when an asymptomatic cancer is identified through medical technology, but the cancer is either non-invasive or so slow-growing that it would never cause medical problems or be life-threatening. In some cases, an asymptomatic, malignant tumor can regress spontaneously—without medical treatment. The diagnosis of cancer is a frightening event, and, instead of postively impacting mortality, treating overdiagnosed cancers with invasive surgery, radiation, and/or chemotherapy can lead to detrimental side effects. Treatment is uneccessary for overdiagnosed cancers and can lead to physical, psychological, and economic harms.
How do we balance the benefits of cancer screening with the harms of overdiagnosis?
One approach is to remove the label of cancer from the low-risk (overdiagnosed) conditions. For example, thyroid cancer incidence has been on the rise in North American for decades, but mortality rates have remained stable—a discrepancy indicative of high percentages of overdiagnosis. Last week’s article in JAMA Oncology (abstract below) presents a recent revision in nomenclature for an indolent thyroid carcinoma, encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC), to a non-malignant condition. Currently most patients with EFVPTC are treated as having an aggressive thyroid cancer. After conducting an international, retrospective study of EFVPTC patients, a panel of endocrinologists 1) reclassified EFVPTC to a non-malignant condition: non-invasive follicular thyroid neoplasms with papillary-like nuclear features or NIFTP; and 2) developed consensus-based, histopathologic diagnostic criteria to appropriately distinguish NIFTP from malignant thyroid cancer. The study authors have coordinated international support for the proposed declassification from professional societies and are working to publicize both the name and diagnostic criteria among clinicians and pathologists.
What does this mean for cancer surveillance?
We will see a decline in thyroid cancer incidence for cases diagnosed in 2016 forward—how rapid a decline will depend upon how quickly the new diagnostic criteria are adopted by clinicians and how our coding system adapts to reflect the new designation. Below is a rough assessment of the changes we might see. Using histology codes 8335/3 Encapsulated follicular carcinoma, 8343/3 Encapsulated papillary carcinoma, and 8340/3 Papillary carcinoma, follicular variant to approximate NIFTP, we see a 30% decrease in thyroid cancer for diagnosis year 2013 due to the change in classification. And while thyroid incidence is still statistically increasing an average of 5.7% per year, based on this NIFTP estimate, more specific codes for NIFTP may result in a further decline in thyroid cancer incidence trends.
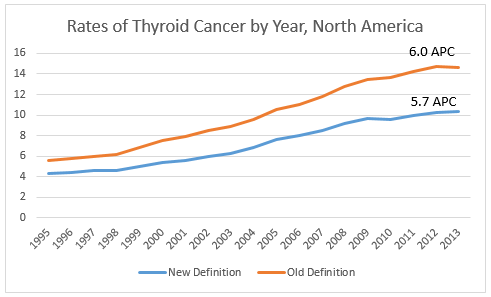 In North America, reportablility follows the guidelines established by the WHO. We will be monitoring WHO “blue books” to see if this reclassification is officially adopted. In order to effectively track the burden of thyroid cancer, the cancer surveillance community will need to ensure our current classification system can accommodate this change, develop guidelines for assessing thyroid cancer trends over time, and educate the public about what is driving the significant drop in thyroid cancer incidence we will see in the new few years.
In North America, reportablility follows the guidelines established by the WHO. We will be monitoring WHO “blue books” to see if this reclassification is officially adopted. In order to effectively track the burden of thyroid cancer, the cancer surveillance community will need to ensure our current classification system can accommodate this change, develop guidelines for assessing thyroid cancer trends over time, and educate the public about what is driving the significant drop in thyroid cancer incidence we will see in the new few years.
Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma (The abstract below is from JAMA Oncology)
Abstract
Importance
Although growing evidence points to highly indolent behavior of encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC), most patients with EFVPTC are treated as having conventional thyroid cancer.
Objective
To evaluate clinical outcomes, refine diagnostic criteria, and develop a nomenclature that appropriately reflects the biological and clinical characteristics of EFVPTC.
Design, Setting, and Participants
International, multidisciplinary, retrospective study of patients with thyroid nodules diagnosed as EFVPTC, including 109 patients with noninvasive EFVPTC observed for 10 to 26 years and 101 patients with invasive EFVPTC observed for 1 to 18 years. Review of digitized histologic slides collected at 13 sites in 5 countries by 24 thyroid pathologists from 7 countries. A series of teleconferences and a face-to-face conference were used to establish consensus diagnostic criteria and develop new nomenclature.
Main Outcomes and Measures
Frequency of adverse outcomes, including death from disease, distant or locoregional metastases, and structural or biochemical recurrence, in patients with noninvasive and invasive EFVPTC diagnosed on the basis of a set of reproducible histopathologic criteria.
Results
Consensus diagnostic criteria for EFVPTC were developed by 24 thyroid pathologists. All of the 109 patients with noninvasive EFVPTC (67 treated with only lobectomy, none received radioactive iodine ablation) were alive with no evidence of disease at final follow-up (median [range], 13 [10-26] years). An adverse event was seen in 12 of 101 (12%) of the cases of invasive EFVPTC, including 5 patients developing distant metastases, 2 of whom died of disease. Based on the outcome information for noninvasive EFVPTC, the name “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP) was adopted. A simplified diagnostic nuclear scoring scheme was developed and validated, yielding a sensitivity of 98.6% (95% CI, 96.3%-99.4%), specificity of 90.1% (95% CI, 86.0%-93.1%), and overall classification accuracy of 94.3% (95% CI, 92.1%-96.0%) for NIFTP.
Conclusions and Relevance
Thyroid tumors currently diagnosed as noninvasive EFVPTC have a very low risk of adverse outcome and should be termed NIFTP. This reclassification will affect a large population of patients worldwide and result in a significant reduction in psychological and clinical consequences associated with the diagnosis of cancer.
The opinions expressed in this article are those of the authors and may not represent the official positions of NAACCR.
